About BioPoly®
Committed To Excellence in Orthopedic Innovation
At BioPoly, we’re dedicated to revolutionizing orthopedic care by advancing materials to improve patient outcomes. Our mission is centered around providing innovative biomaterial implants designed to restore natural joint function, relieve pain, and help patients return to their active lifestyles.

Our Story
In 2004, Herb Schwartz set out to revolutionize the way orthopedic innovations transition from concept to reality. Herb, a Ph.D. in Biomechanical Engineering and a prolific inventor with over 50 U.S. patents, has a talent for identifying unmet needs in orthopedics and developing innovative solutions. Sheila, a pharmacist with unmatched organizational skills and expertise in strategy, leadership, and execution, complements his vision perfectly. Together, they founded Schwartz Biomedical—BioPoly’s parent company—and later, BioPoly itself. Sheila’s involvement in the beginning was as the Company’s first angel investor, funding the operation so that Herb could focus on getting everything started. Sheila later joined BioPoly full time in 2013 and is now BioPoly’s Chief Operating Officer and heads up manufacturing, clinical/regulatory, accounting and finance. Herb is BioPoly’s President and CEO, and both Herb and Sheila serve on the Board of Directors.
The journey began in Fort Wayne, Indiana, at the Northeast Indiana Innovation Center (NIIC), a business incubator that provided crucial early-stage support which helped position Schwartz Biomedical for success as a launchpad for groundbreaking orthopedic technologies.

From Incubator to Industry Impact
Schwartz Biomedical operates as an orthopedic incubator, developing novel technologies and transitioning them into standalone companies. The first spin-off was BioDuct, a meniscal repair technology company that achieved FDA clearance and was sold to a major orthopedic company within three years. Their second spin-off, BioPoly, became their most challenging and rewarding endeavor to date.
BioPoly centers on a revolutionary biomaterial designed for orthopedic applications. The original concept—combining hyaluronic acid with polyethylene to create a superior bearing material—emerged from research led by Professor Sue James at Colorado State University. Recognizing the potential, Schwartz Biomedical licensed the technology and secured grant funding from Indiana’s 21st Century Fund to refine and optimize the material.
As Herb pitched the BioPoly vision to the 21st Century Fund committee, Dr. Dane Miller, founder of Biomet, took him aside and said, “If you can make this work, it will change everything in orthopedics.” With over $3 million in grant funding, the team began the demanding work of turning this vision into reality.

Breakthroughs in Material Science and Clinical Adoption
A key breakthrough was solving the complex challenge of manufacturing BioPoly’s unique material. Years were spent developing proprietary techniques to ensure the BioPoly material with its cartilage-like lubricity would still have the strength of traditional orthopedic polyethylene (UHMWPE) so that it could carry the demanding joint loads – not a trivial task. To meet stringent regulatory requirements in orthopedics, the company achieved ISO 13485 certification in 2009 and has maintained it ever since. Also, in order to thrive in this industry, the Company’s infrastructure had to be rock solid and well-organized.
Along the way, the team made a game-changing discovery: BioPoly could articulate with cartilage without causing damage—a feat unattainable with traditional polyethylene due to its hydrophobic properties. The addition of hyaluronic acid transformed BioPoly into a material capable of partial joint resurfacing, a less invasive solution than total joint replacements.

Expanding Global Reach and U.S. Market Growth
In 2011, BioPoly introduced its first implant, the BioPoly Partial Resurfacing Knee, to the European market via CE Mark certification. In 2012, the first patient received a BioPoly Knee Implant in London, England—and that patient still has the implant today! Since then, the company has launched additional CE-marked products, including the BioPoly Patella, Trochlea, and Shoulder implants. Two clinical studies in Europe have demonstrated the implants’ success, with 2- and 5-year results published in the Journal of Bone and Joint Surgery.
In 2016, the company outgrew the NIIC incubator and moved to its current headquarters in Fort Wayne, Indiana. The next chapter in BioPoly’s journey is focused on the U.S. market. The company achieved Breakthrough Device Designation in 2023 from the FDA for its BioPoly Knee Implant and gained approval to initiate a clinical study in the U.S., which is currently underway. Beyond the knee, BioPoly has developed and received FDA clearance for additional products, including the BioPoly Great Toe, Lesser Toe, and Radial Head Implants. Many more BioPoly products are in the development pipeline, including the original application for a superior total joint material. Stay tuned for more innovation coming from BioPoly in the near future.
Our Values
BioPoly operates with a commitment to core values that drive everything we do:
Looking To The Future
We are excited to continue advancing our technology to provide innovative solutions that help preserve mobility, reduce pain, and improve recovery times. The future of joint repair is bright, and BioPoly is leading the way.
Laura H. — BioPoly Patient
Explore Our Range of BioPoly® Implants
Innovative Solutions for Joint Repair
BioPoly offers a range of advanced orthopedic implants designed to replace cartilage. Each product is manufactured from our patented, self-lubricating material, providing an early-stage solution for patients to regain pain-free movement and restore their active lifestyles. Explore our product lineup to find the right solution for your needs.
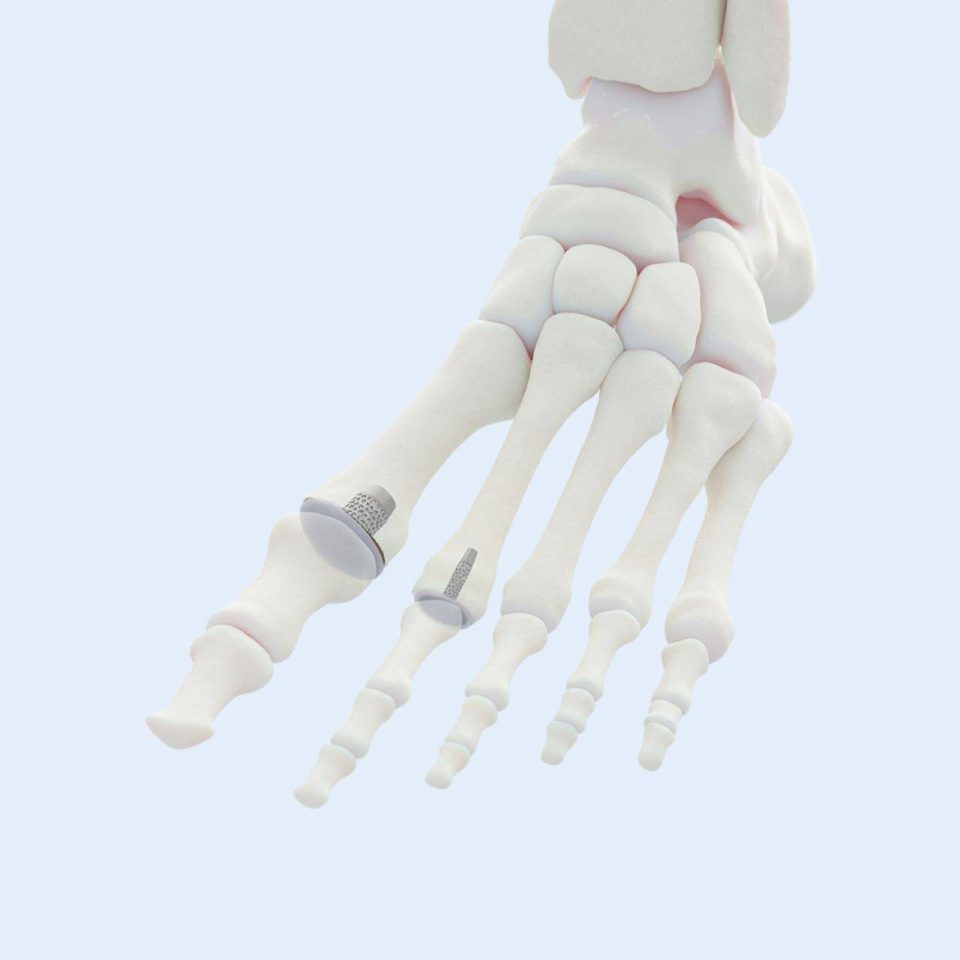
Great & Lesser Toe Implants
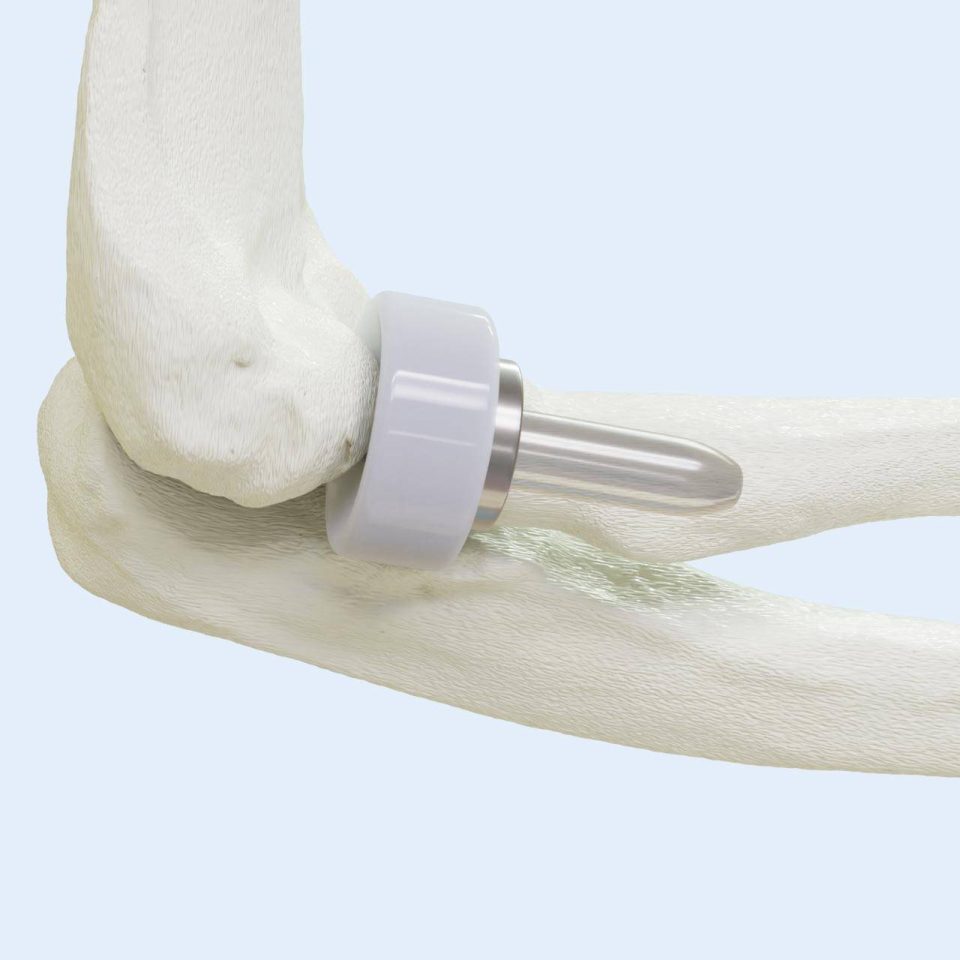
Radial Head Implant

Knee Implant

Products Pipeline
