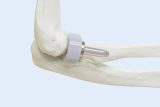
BioPoly® Granted US Patent for Its Implant Technology
General
March 6, 2020
FORT WAYNE, IN – BioPoly LLC has announced that another U.S. patent has been granted for its partial resurfacing implants which protects the innovative implant designs. The Company’s intellectual property portfolio also consists of patents that address its unique BioPoly® material. This most recent patent further adds to the Company’s IP portfolio, providing assurance that all aspects of the implants, material, and surgical technique are protected throughout the world.
Utilizing simple, reproducible minimally invasive surgical techniques, the novel BioPoly® resurfacing implants are designed to replace only the damaged cartilage of a patient’s joint rather than to replace the entire joint. The BioPoly® material used in the implants is a proprietary combination of hyaluronic acid and ultra-high molecular weight polyethylene, creating a self-lubricating polymer with enhanced wear properties. Over eight years of clinical experience shows that BioPoly implants allow patients to quickly return to active, pain-free lifestyles. The current BioPoly product offering consists of the RS Knee, RS Trochlea, RS Patella, and RS Shoulder implants. The implants are CE marked and currently available throughout Europe.
About BioPoly, LLC
BioPoly LLC is an ISO 13485 certified orthopaedic implant manufacturer located in Fort Wayne, Indiana. The Company is developing, manufacturing and marketing products for use in sports medicine and orthopaedics. Additional medical applications of the BioPoly® technology in cardiovascular, spinal, and trauma markets are also being pursued.