News
Get The Latest Information From BioPoly

General
BioPoly Welcomes Scott Bowles to Board of Directors
April 25, 2025
Apr
25
2025
Press Release
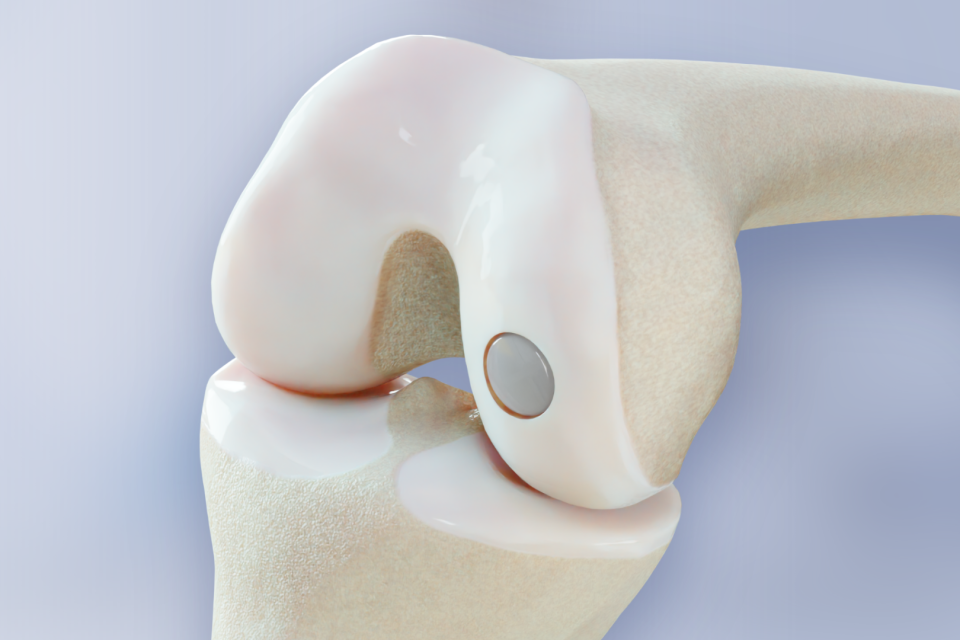
BioPoly® Knee FDA-Approved Clinical Study is Now Published on ClinicalTrials.gov
April 22, 2025
Apr
22
2025

Founder's Facts
Founder’s Facts: What Makes BioPoly®… BioPoly?
April 4, 2025
Apr
4
2025

Press Release
BioPoly LLC Expands Intellectual Property Portfolio with Newly Granted U.S. Patent for Shoulder Implant
March 12, 2025
Mar
12
2025
Founder's Facts
Founder’s Facts: Patient Selection
February 21, 2025
Feb
21
2025
Press Release
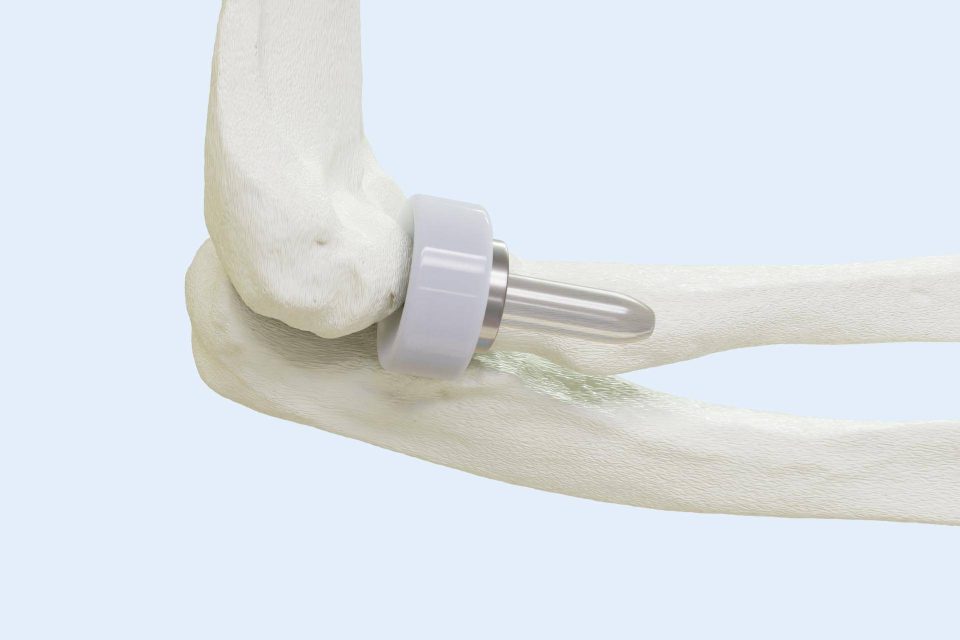
BioPoly LLC Announces First-Ever Elbow Surgery with Revolutionary BioPoly® Radial Head Implant
February 13, 2025
Feb
13
2025

Founder's Facts
Founder’s Facts: What is BioPoly®?
January 10, 2025
Jan
10
2025
Founder's Facts
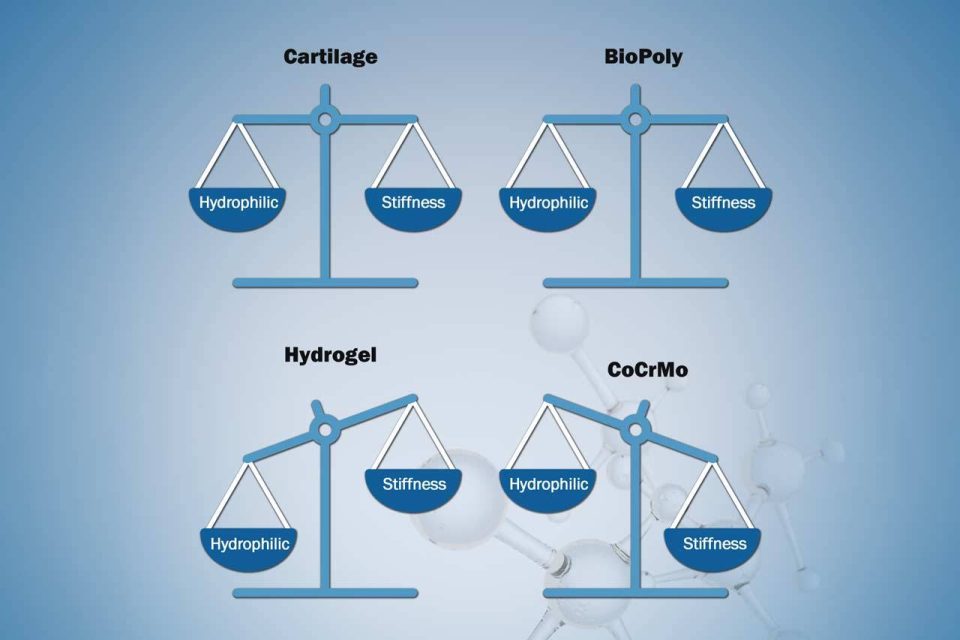
Founder’s Facts: BioPoly vs Hydrogels
December 6, 2024
Dec
6
2024

Founder's Facts
Founder’s Facts: BioPoly and Cartilage – Benefits of a New Biomaterial – Part 2
November 15, 2024
Nov
15
2024

Founder's Facts
Founder’s Facts: BioPoly and Cartilage – Benefits of a New Biomaterial
November 1, 2024
Nov
1
2024

General
Founders Facts – BioPoly and Cartilage
October 28, 2024
Oct
28
2024

General
BioPoly® Radial Head System Receives FDA Clearance
March 14, 2024
Mar
14
2024