News
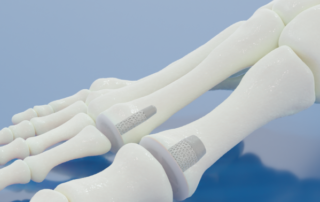
BioPoly® Announces FDA Clearance of Its Lesser Toe Implant
FORT WAYNE, IN – BioPoly LLC has announced that the U.S. Food and Drug Administration has granted clearance for its BioPoly® Lesser Toe System, BioPoly’s second FDA-cleared product in the U.S. market.BioPoly’s Great Toe System was the first product to receive FDA clearance, which is currently being sold in the US for patients with arthritis leading to a stiff or painful great toe. “We are excited about achieving our second FDA clearance with BioPoly technology as we continue to build momentum in our extremity portfolio. The Great Toe System is performing well clinically and we expect the same from our [...]
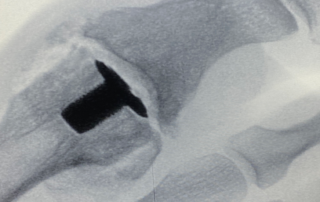
First BioPoly® Great Toe Surgery Performed in US
FORT WAYNE, IN – BioPoly LLC has announced that the first BioPoly® Great Toe surgery in the U.S. was completed on September 30, 2021. The surgery was performed by Dr. Steve Herbst of Central Indiana Orthopedics in Muncie, Indiana.“The BioPoly Great Toe implant addresses a large unmet need in the market, and we are thrilled to have it available in the U.S. now. This small implant has a lot of technology packed into it and solves many of the issues that have caused other great toe implants to fail,” said Herb Schwartz, President of BioPoly. The BioPoly® Great Toe implant [...]
BioPoly® Announces FDA Clearance of Its Great Toe Implant
FORT WAYNE, IN – BioPoly LLC has announced that the U.S. Food and Drug Administration has granted clearance for its BioPoly® Great Toe Implant. With this FDA clearance, the BioPoly technology is available for the first time in the U.S. BioPoly first received regulatory approval in 2012 for its knee implants in Europe. “Although we have been in patients in Europe with our knee implants for nearly 10 years, we are extremely excited to be able to enter the US market with our BioPoly technology,” said Herb Schwartz, President of BioPoly. “We have a porous and non-porous stem design and [...]
BioPoly® Orthopedic Resurfacing Products Enter North America
FORT WAYNE, IN – BioPoly, LLC has entered into an exclusive distribution agreement with Canada-based distributor, Verve Medical Products, Inc., to distribute the BioPoly® partial resurfacing family of products throughout Canada, with distribution initially taking place through the Canadian SAP. Verve Medical Products specializes in bringing medical-surgical products to Canada through its distribution and regulatory services. Herb Schwartz, BioPoly President, said “The introduction of BioPoly’s product portfolio into North America, specifically the Canadian market, is a major accomplishment for our Company. Verve Medical Products is one of Canada’s leading medical device distributors, and we are looking forward to working with [...]
BioPoly® Granted an International Patent for Its Implant Technology
FORT WAYNE, IN – BioPoly LLC has announced that the Company has further expanded its intellectual property portfolio. The Company was granted another international patent for its partial resurfacing implants which protects the innovative implant designs. In addition to patents that cover the design of its implants, several U.S. and international patents have also been granted that address the Company’s unique BioPoly® material. The Company’s IP portfolio, including this most recent patent, provides assurance that all aspects of its implants, material, and surgical technique are protected throughout the world. Rather than replacing the entire joint, the novel BioPoly® resurfacing implants [...]