BioPoly Partial Resurfacing Knee
*IDE Approved
CAUTION: Investigational Device. Limited by Federal Law (United States) to investigational use only. (21 CFR 812.5(a))
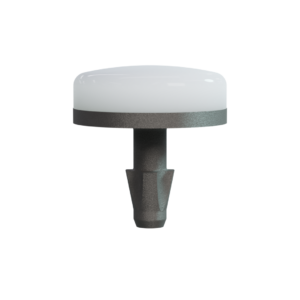
115-5002 15mm BioPoly® Partial Resurfacing Knee (Distal Femur)
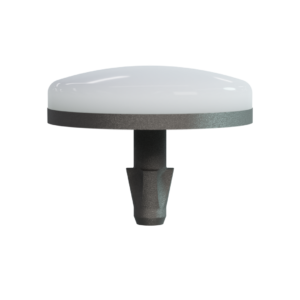
115-5003 20mm BioPoly® Partial Resurfacing Knee Implant (Distal Femur)

115-5004 15x24mm BioPoly® Partial Resurfacing Knee Implant (Distal Femur)