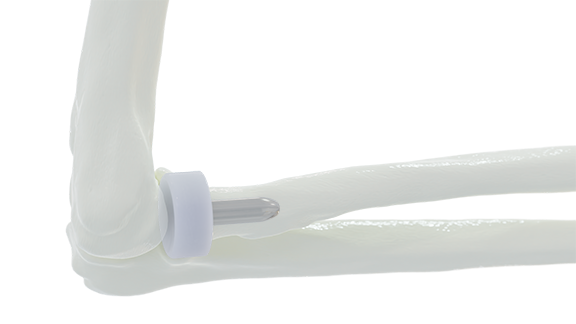
 FORT WAYNE, IN – BioPoly LLC announced today that the FDA has granted clearance on their new BioPoly® Radial Head System for the elbow. This implant is manufactured from BioPoly’s proprietary material and is the only synthetic cartilage Radial Head on the market.
FORT WAYNE, IN – BioPoly LLC announced today that the FDA has granted clearance on their new BioPoly® Radial Head System for the elbow. This implant is manufactured from BioPoly’s proprietary material and is the only synthetic cartilage Radial Head on the market.
With this FDA clearance, BioPoly has taken significant steps toward achieving its strategy of expansion of its orthopedic, resurfacing implants in the US. The team has been actively developing multiple implant systems for many years as we worked toward gaining FDA clearance in the US market. Dr. Herb Schwartz, BioPoly’s Chairman and CTO, said, “We now have 3 FDA cleared products in 3 different joints and we also have an FDA Master File for the material. The BioPoly Radial Head clearance shows that our synthetic cartilage platform has been accepted by the FDA and the market. We have many more implant systems that are at various stages within the regulatory approval process; so, stay tuned as we continue to expand our implant offerings.”
“FDA clearance of the BioPoly Radial Head is a major accomplishment for our company, and I am very proud of our team which consists of surgeons, engineers, scientists and operations experts. It takes the entire team to develop an implant of this caliber which will change the landscape of radial head surgery because surgeons now have a synthetic cartilage option for their patients,” said Ryan Schlotterback, BioPoly’s President & CEO, “Since Radial Head implants articulate with patients’ natural cartilage, BioPoly is perfect for this application. We will be launching the system very soon. The surgeon team who helped develop this system and surgeons who have already been trained are very excited to have the BioPoly Radial Head available to them.”
The Radial Head market is full of metal implants which are known to wear away the opposing cartilage in the elbow joint; however, with the BioPoly Radial Head, surgeons have a better long-term solution for their patients. The BioPoly material has been used clinically in multiple joints (knee, patella, trochlear groove, great toe, and lesser toes) for over 12 years and has repeatedly proven that it does not damage the opposing cartilage surface like metal implants.
BioPoly LLC is an ISO 13485 certified orthopedic implant manufacturer located in Fort Wayne, Indiana. The Company develops, manufactures, and markets orthopedic implants that use its proprietary BioPoly material to replace damaged cartilage in joints. BioPoly currently has multiple FDA cleared products and is actively developing additional products for the US market. Visit our website: www.biopolyortho.com
